China steps up readiness to handle logistical complexities of global COVID-19 vaccine distribution

The cargo shipment of COVID-19 vaccines produced by the Chinese company Sinovac Biotech is being dispatched at the Cumbica airport in Guarulhos, Sao Paulo state, Brazil, on July 20. Photo: AFP
With
the coronavirus vaccine possibly ready for public use as early as November, the
rapid rollout of the vaccine candidates still poses great logistical challenges
in global vaccine transportation.
Though China has not yet exported COVID-19 vaccine candidates for non-clinical
trials use, all stakeholders in the supply chain, from vaccine producers to
relevant departments and logistics providers have been fully prepared for
global transportation to meet unprecedented challenges. International
organizations promoting equal vaccine distribution around the world have also
shown support to cope with potential threats.
Working on shipping schedule
China has promised to give the priority access to a vaccine to a list of
countries, mostly in Southeast Asia, Africa and Latin America. Many countries
may have to rely on China for global transportation of the COVID-19 vaccine,
considering its advanced equipment and more developed logistics system. China's
three leading coronavirus vaccine providers - Sinopharm, CanSino, and Sinovac -
are working out detailed plans for international procurement and transportation
with relevant authorities, a senior expert working with SF Express, who is
charge of COVID-19 vaccine global logistics but prefers not to be named, told
the Global Times on Monday.
The top vaccine producer Sinopharm has so far received COVID-19 vaccine orders
for more than 500 million doses, but its current production capacity cannot
satisfy all the orders, said the anonymous expert. Shortages of packaged
materials such as glass bottles and rubber plugs are also limiting the speed at
which Sinopharm can currently export its vaccines.
To save packaging materials, another frontrunner company CanSino will export
the raw pulp used to develop the COVID-19 vaccine in cans instead of directly
exporting the packaged vaccine, according to the senior expert.
China's vaccine suppliers and logistics companies are consistently working on
plans for international transportation routes in recent weeks, and are in close
communication with relevant international organizations and airlines. Chinese
vaccine manufacturers are also actively seeking export licenses from the
Chinese government.
International shipments of Chinese-produced vaccines to overseas markets are
expected to begin as early as mid-December. The transportation plan should be
specified at least one month in advance in order to settle down the financial
bill and equipment purchase, the expert added.
"For example, the cost of transporting vaccines to Brazil on charter
flights is about 7 million yuan ($1.05 million), so it is likely airlines will
stop at the transfer station and replenish other sources in transit to balance
economic costs. But stopping is a greater risk for the vaccine, requiring more
professional equipment," said the expert.
The World Health Organization estimates up to 50 percent of vaccines may be
wasted globally every year due to temperature control, logistics and
shipment-related issues.
"COVID-19 vaccine distribution will be unlike any previous
logistical challenge. It will need to reach nearly 200 countries in a
relatively short timeframe and will need to move in a safe and secure manner to
ensure full compliance with cool chain temperature requirements," Glyn
Hughes, head of cargo at the International Air Transport Association, told the
Global Times in a recent interview.
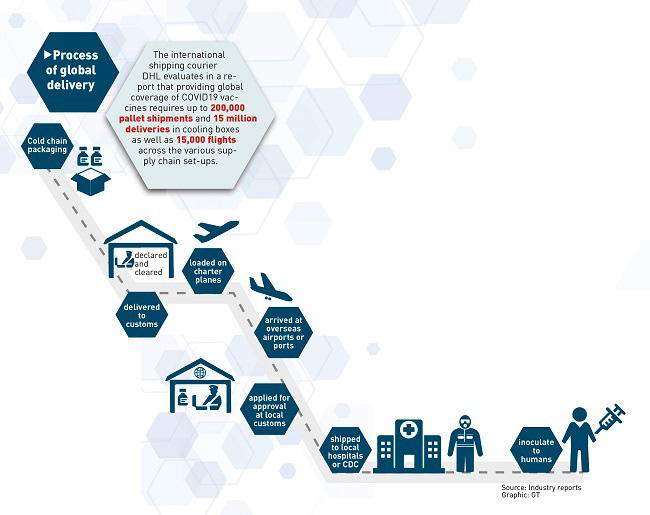
Other
difficulties lie in shortages of supporting facilities and high cross-border
transport costs. The international shipping courier DHL evaluated in a report
that providing global COVID-19 vaccine coverage requires up to 200,000 pallet
shipments and 15 million deliveries in cooling boxes, as well as 15,000 flights
across supply chain set-ups.
As the transportation trading volume of vaccines increases, international
freight market prices may surge when vaccines start to be shipped. But given
the pandemic, customs clearance approvals are either stagnant or slowing down
in many countries. This exacerbates the difficulty, Chen Yong, manager of the
Lengwang Technology Co., which provides cold chain solutions for traditional
medical products logistics, told the Global Times.
The cold-chain challenge
A lack of a certain assessment of the COVID-19 vaccine's thermal stability
could mean more rigorous temperature control along the vaccine supply chain,
with certain types of coronavirus vaccine (mRNA) requiring storage in
facilities as low as -70 degrees Celsius, while some other types - such as
inactivated vaccines - need temperatures at the conventional range of 2-8
degrees Celsius.
Overheating may compromise the vaccine or cause adverse reactions and even
death to the human body without clear evaluation.
"The air cargo supply chain is complex in normal conditions. When you
then include the additional challenges of cool chain requirements associated
with least developed countries, that complexity is magnified," said Glyn
Hughes. "Airport infrastructure is one area that will require close
scrutiny to ensure that vaccines are flown into appropriate facilities.
In-country supply chains will also need to be upgraded in many developing
nations to ensure the temperature integrity is maintained throughout the
journey from production to injection."
The freezing package of the vaccine is usually effective for five days during
transnational journey which takes at least two or three days from packing to
landing. If transit is involved, it will take longer, and bring a bigger
challenge for temperature-monitoring systems and storage space at the transit
station, Chongqing Zhifei Biological Products, a company manufactures and sells
vaccines and biological products, told the Global Times.
Emirates SkyCargo announced to have the world's first dedicated airside cargo
hub for the vaccine in Dubai on October 23.
Emirates SkyCentral DWC has more than 4,000 square meters set aside for
temperature-controlled GDP-certified dedicated pharmaceutical storage, allowing
for large-scale storage and distribution of the potential COVID-19 vaccines.
Overall, it is estimated that the facility can hold around 10 million vials of
vaccine at a range of 2-8 degrees Celsius temperature at any one point of time,
the airline company suggested in a statement sent to the Global Times.

A staff member works during a media tour of a new factory built to produce a COVID-19 vaccine at Sinovac, one of 11 Chinese companies approved to carry out clinical trials of potential coronavirus vaccines in Beijing on September 24. Photo: AFP
Global
support
As part of global efforts to strengthen vaccine supply chains and achieve
better immunization equity and coverage, in 2015, the Vaccine Alliance (GAVI),
UNICEF and other partners launched the Cold Chain Equipment Optimization
Platform (CCEOP) - a $400 million, five-year project to upgrade existing cold
chain equipment in 56 countries by 2021. Strengthening cold chains also
supports agile vaccine response efforts for infectious disease outbreaks,
including the COVID-19 vaccine when it becomes available, according to UNICEF.
"The key constraint in 2021 will not be potential intellectual property
dispute, but rather supply constraints, and addressing these issues will
require utilizing all available expertise and resources - including that of
industry," GAVI spokesperson told the Global Times. So far, the
organization has already secured up to 200 million doses of a vaccine for
lower-income countries.
"Technology transfer can succeed only when the innovator company works
closely with the recipient in order to replicate the complex manufacturing
processes in the new facility, and both partners are committed to overcoming
the many challenges that can emerge. Consequently, any partnerships in pursuit
of this goal must be viewed as advantageous from both sides," the
spokesperson said.
Sheikh Abdullah bin Mohammed al-Hamed, chairman of the Department of Health of UAE, is undergoing a clinical trial for the third phase of the inactive vaccine for COVID-19 in Abu Dhabi. Photo: AFP


